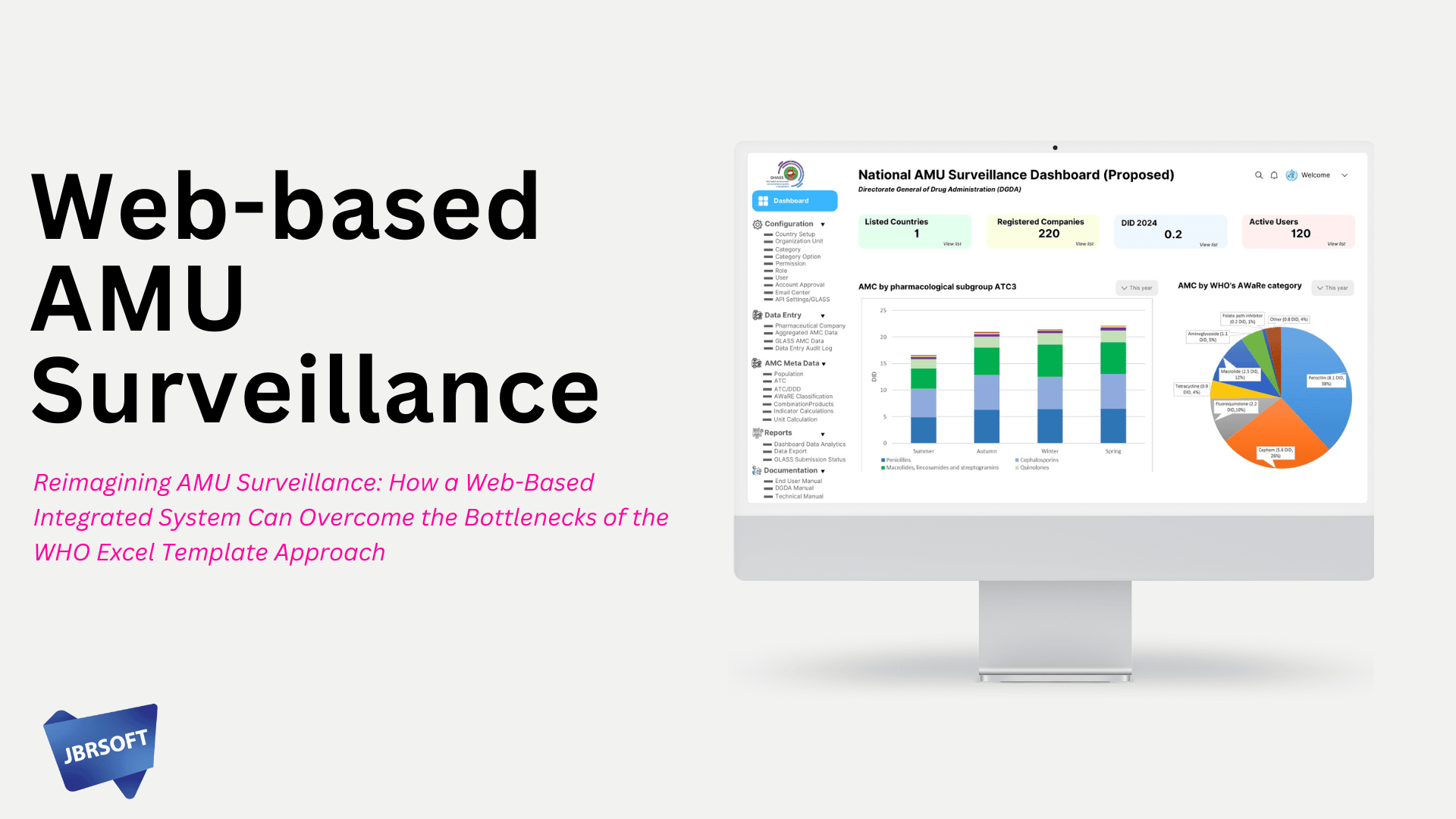
Antimicrobial Use (AMU) surveillance plays a critical role in global efforts to combat antimicrobial resistance (AMR). While the WHO-provided Excel-based AMU Surveillance Template has been instrumental in initiating standardized national data collection, it brings significant logistical and analytical challenges—particularly in large-scale, recurring data collection from pharmaceutical companies. As we face rising demand for accuracy, efficiency, and timely reporting, a web-based integrated AMU surveillance system offers a forward-looking solution that addresses existing bottlenecks and enhances data utility.
Key Challenges of the Excel-Based System
Before delving into the web-based system, it's vital to understand the systemic bottlenecks in the current Excel-driven model:
- Manual Data Collection & Distribution: The need to circulate the Excel macro template to dozens or hundreds of companies biannually is a resource-intensive task.
- Data Integration Pain Points: Consolidating diverse spreadsheets from multiple entities into one national dataset involves significant manual effort.
- Error-Prone Workflows: Manual validations increase the likelihood of inconsistent formats, calculation errors, and duplication.
- Delayed Reporting: Manual GLASS export preparation and consumption calculations delay timely submission to global surveillance platforms.
- Lack of Real-Time Insights: With no dynamic dashboards or automated analytics, data interpretation remains slow and reactive.
Why a Web-Based Integrated AMU Surveillance System?
A centralized web-based AMU surveillance platform solves these issues with automation, standardization, and real-time oversight.
1. Centralized Data Submission by Stakeholders
Pharmaceutical companies securely log in to upload their antimicrobial sales or import data directly into the system using standardized digital forms or spreadsheet uploads. This eliminates the need to circulate Excel files for every reporting period.
2. Automated Validation Rules
Built-in validation mimics the Excel macro functions: e.g., product verification, calculation of Defined Daily Doses (DDD), and alignment with ATC/DDD databases. This reduces manual error-checking time and ensures consistency across submissions.
3. Real-Time Data Processing and Standardization
Automatic ingestion and processing of product-level data, including salt forms, strength, dosage, route of administration (RoA), and pack sizes. Lookup tables for ATC classification, DDD conversions, units, and product metadata mirror WHO’s macro template structure but in dynamic form.
4. Dynamic Dashboards and Analytics
Role-based dashboards allow national surveillance units to visualize trends by sector, product class, import/local production, and consumption patterns over time—no post-hoc Excel manipulation required.
5. Automated GLASS Export and Submission
Seamless generation of WHO GLASS AMC-compatible output formats for submission ensures timely reporting and compliance with global reporting cycles.
6. Controlled User Access & Workflow Management
Role-based accounts (e.g., Pharma, Reviewer, Admin) streamline workflow from data entry to final approval. Automated logs and notifications enhance traceability and accountability.
7. Scalable and Adaptable Infrastructure
The system can be scaled to accommodate additional modules such as hospital AMU, veterinary medicine, and environmental surveillance. This aligns with the One Health framework and enhances long-term sustainability.
🚀 From Manual to Intelligent: The Path Forward
Moving from manual Excel-based workflows to a web-based integrated AMU surveillance platform is not merely a technical upgrade—it’s a systemic shift. It empowers national health authorities with:
- Efficiency in operations
- Accuracy in reporting
- Speed in analysis
- Scalability for future AMR surveillance expansion
By automating the backbone of WHO’s surveillance template and embedding it into a secure web system, countries can leapfrog operational bottlenecks and align better with the One Health vision of integrated AMU/AMR monitoring.
🖋️ Written by:
The JBRSOFT AMR Informatics Team
Empowering Health Systems through Digital Innovation
Follow Our WorkTo stay informed about our latest innovations in health systems and digital transformation, follow JBRSOFT Limited on:
🌐 Website: https://jbrsoft.com
🔵 LinkedIn: linkedin.com/company/jbrsoft
📘 Facebook: https://www.facebook.com/jbrsoft
🐦 Twitter: https://x.com/Jbrsoft10
If you are interested in a similar business case, feel free to contact us: Email: management[@]jbrsoft.com, Direct: +8801968-192627.
#Bottlenecks #SystemicChallenges #AMUSurveillance #AMR #GLASS #PublicHealth #OneHealth #WHO #HealthData #AMRContainment #OHASS #DGDA #AMRBD #JBRSOFT