While the WHO’s Excel-based Antimicrobial Use (AMU) Surveillance Template provides a structured approach to collecting national-level consumption data, it is not without critical limitations. For countries managing data from hundreds of pharmaceutical companies, relying on a manual, Excel-driven system can significantly impede data quality, timeliness, and efficiency.
This article outlines the major bottlenecks and systemic challenges associated with Excel-based AMU surveillance and highlights the need for a centralized, web-based digital platform.
1. Circulating the Template to Dozens or Hundreds of Companies
Disseminating the AMU Excel template to each pharmaceutical manufacturer or importer, whether bi-annually or annually, presents logistical difficulties:
- Requires manual coordination with all data providers
- Risks version mismatches and inconsistencies
- Causes delays in data submission and stakeholder engagement
2. Manual Data Collection at Scale is Inefficient
Each company must manually fill in the template with product-wise consumption or sales data. This presents issues such as:
- Inconsistent formats across submitted files
- Missing or incomplete fields
- Dependency on individuals’ spreadsheet skills
- Poor version control and data consolidation fatigue
3. Data Consolidation is Labour-Intensive
After receiving data from multiple entities, the national surveillance focal point or coordinating authority must:
- Merge data from different Excel files
- Match products to WHO ATC codes and standardize units
- Clean inconsistencies across entries
- Deduplicate product records
4. Data Cleaning & Harmonization is Manual
Excel does not inherently support intelligent validation. This leads to:
- Manual detection of anomalies, such as incorrect ATC coding, duplicates, or wrong units
- Lack of automatic feedback to contributors for correction
- No version tracking or change audit trails
5. Error-Prone Calculation & DDD Estimation
The macro-enabled template performs basic calculations, but still has several weaknesses:
- Errors in input data cause cascading calculation issues
- DDD estimation can be incorrect due to:
- Improper RoA (route of administration)
- Unaccounted salts or combinations
- No real-time feedback on calculation validity
6. No Automated Dashboard for Visualization
The Excel template is static and lacks interactive data visualization tools. Users must:
- Manually generate pivot tables or charts
- Rely on external tools (e.g., Power BI, Tableau)
- Spend extra time in data slicing for internal review
7. GLASS Export is Not Seamless
Although macros allow for partial export, several issues persist:
- Exported files may not pass WHO GLASS validation on first attempt
- Countries must often edit files manually to meet formatting standards
- No traceability between submitted data and original sources
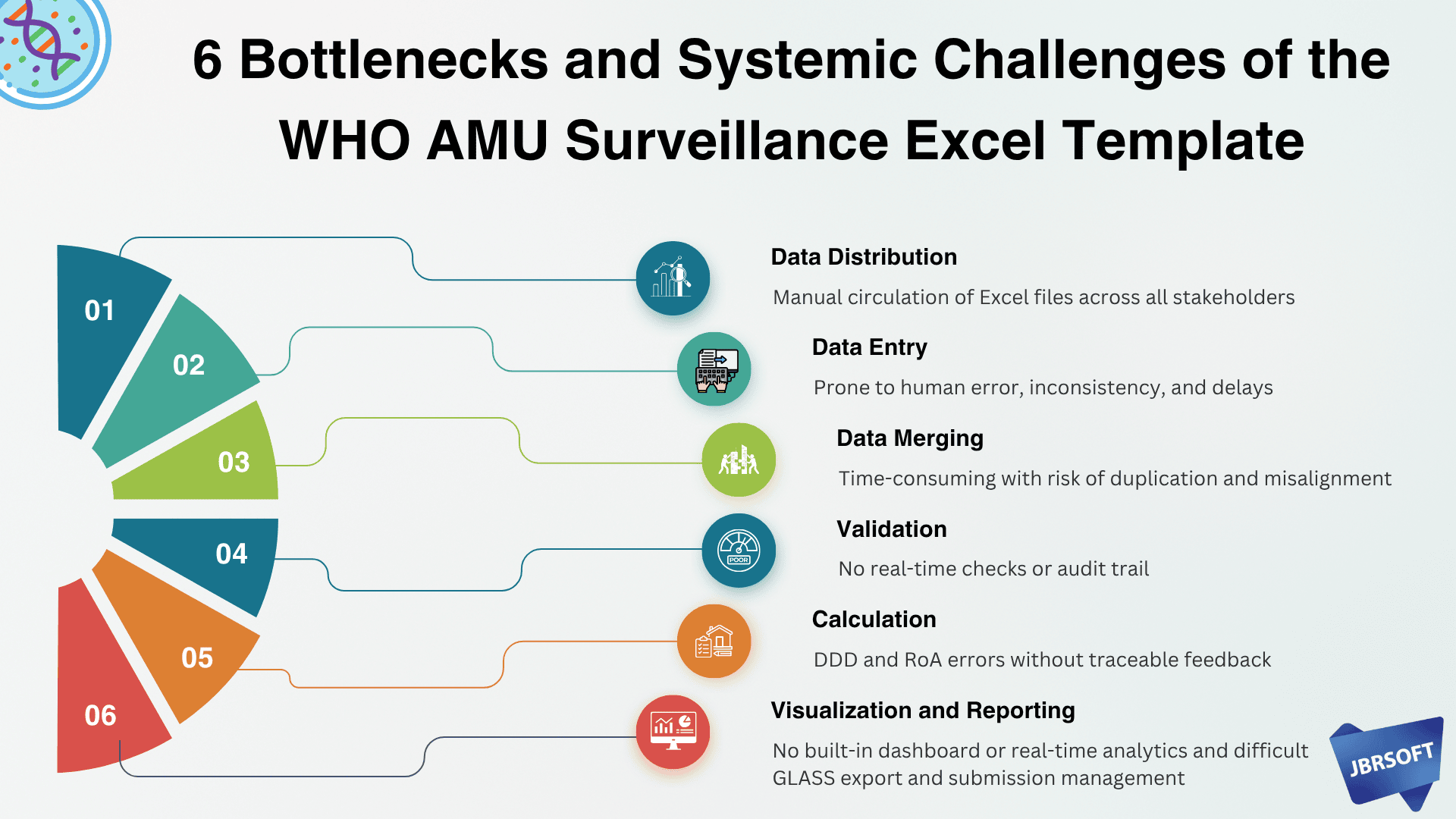
Summary of Limitations
| Area | Key Limitation |
|---|---|
| Data Distribution | Manual circulation of Excel files across all stakeholders |
| Data Entry | Prone to human error, inconsistency, and delays |
| Data Merging | Time-consuming with risk of duplication and misalignment |
| Validation | No real-time checks or audit trail |
| Calculation | DDD and RoA errors without traceable feedback |
| Visualization | No built-in dashboard or real-time analytics |
| Reporting | Difficult GLASS export and submission management |
The Case for a Centralized Web-Based AMU Surveillance Platform
To overcome the Excel template’s limitations, there is a strong case for developing a centralized digital AMU surveillance system that:
- Allows online data entry by pharmaceutical companies through secure logins
- Automates product matching with ATC and WHO definitions
- Enables real-time validation, data cleaning, and quality control
- Facilitates automatic DDD calculations and normalization
- Provides interactive dashboards for internal monitoring
- Generates GLASS-compatible reports seamlessly
- Supports audit trails, version control, and timely reminders
Conclusion
While the WHO Excel template has helped establish a global foundation for AMU surveillance, its limitations are significant in countries with complex pharmaceutical ecosystems. A shift toward a scalable, digital, and intelligent surveillance platform is essential for strengthening national AMU programs and accelerating progress toward global AMR containment.
🖋️ Written by:
The JBRSOFT AMR Informatics Team
Empowering Health Systems through Digital Innovation
Follow Our WorkTo stay informed about our latest innovations in health systems and digital transformation, follow JBRSOFT Limited on:
🌐 Website: https://jbrsoft.com
🔵 LinkedIn: linkedin.com/company/jbrsoft
📘 Facebook: https://www.facebook.com/jbrsoft
🐦 Twitter: https://x.com/Jbrsoft10
If you are interested in a similar business case, feel free to contact us: Email: management[@]jbrsoft.com, Direct: +8801968-192627.
#Bottlenecks #SystemicChallenges #AMUSurveillance #AMR #GLASS #PublicHealth #OneHealth #WHO #HealthData #AMRContainment #OHASS #DGDA #AMRBD #JBRSOFT